Forward-looking: Batteries are typically rigid blocks, which has made designing small, flexible, and wearable electronics around them difficult. Researchers are beginning to tackle the problem by rethinking the elements used to make batteries, resulting in thin, flexible materials that can reliably hold electricity after repeated stretching or compression.
Two groups of researchers recently published studies on the prospect of designing flexible batteries almost simultaneously. Despite being unrelated, the papers detail similar prototypes.
The American Chemical Society reports that a team from Nanjing University devised a lithium-ion battery with components that can stretch to up to 5,000 percent of their original length. Furthermore, the battery remains healthy through about 70 full charge cycles.

According to the researchers, other batteries have tried to achieve flexibility by either folding solid materials like paper or weaving them into conductive fabric. However, these can be difficult to manufacture or lose charge due to components that weaken from repeated stretching and folding.
The researchers solved the problem by going deeper and making every part of the battery elastic. The electrodes crucial to the technology comprise a stack of conductive paste, silver nanowires, carbon black, polydimethylsiloxane, lithium salt, electrode film, and protective coating. A solid rubber-like layer is formed when activated by light.
While there is room for improvement, the material could make wearables and implants better accommodate users' movements. A team from the University of Cambridge published another approach to the problem on the same day as the Nanjing researchers.
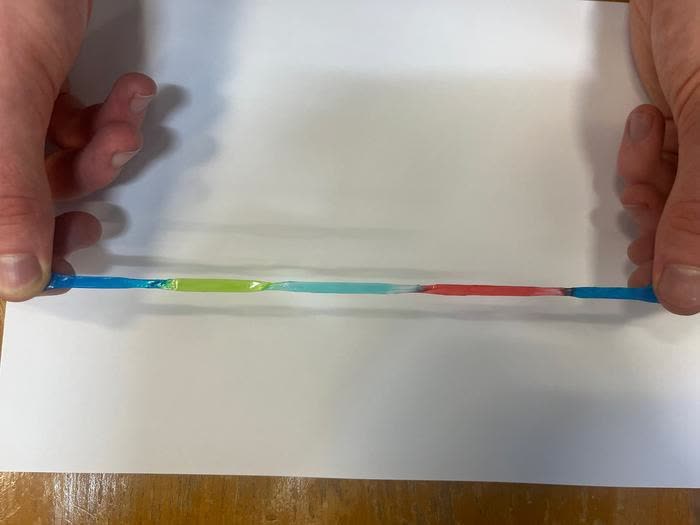
This study resulted in a jelly-like material that can maintain a charge and revert to its original shape after being squashed or stretched to 10 times its original length. The central component – hydrogels – are mostly made of water but exhibit properties enabling the researchers to manipulate their mechanical attributes.
Changing the hydrogels' salt content makes them more adhesive and resilient, increasing their charge and allowing them to stretch without losing charge. The researchers could potentially change the material's properties to match human tissue, making it ideal for powering electrical implants. Tests on living organisms are forthcoming.
The design from the Cambridge study drew significant inspiration from the biological structure of electric eels, which use special organs to shock and stun their prey. Early examinations of electric eels in the 18th century also helped inspire the invention of the first electric battery in 1800.
Image credit: American Chemical Society
